Peripheral neuropathy (PN) is a complex and poorly understood condition in which the body’s peripheral nerves gradually deteriorate (Fig. 1). Symptoms associated with this condition range from mild tingling and numbness to severe throbbing, stabbing and/or burning pain. Intense nerve pain affects as many as two thirds of people afflicted with peripheral neuropathy and the symptoms are notoriously difficult to treat. As the disease progresses, the resultant muscle weakness and impaired sensation leads to a heightened risk of injuries from falls. Unfortunately, peripheral neuropathy is surprisingly common, affecting 10% of middle-aged adults and nearly 40% of people over the age of 70 (1).
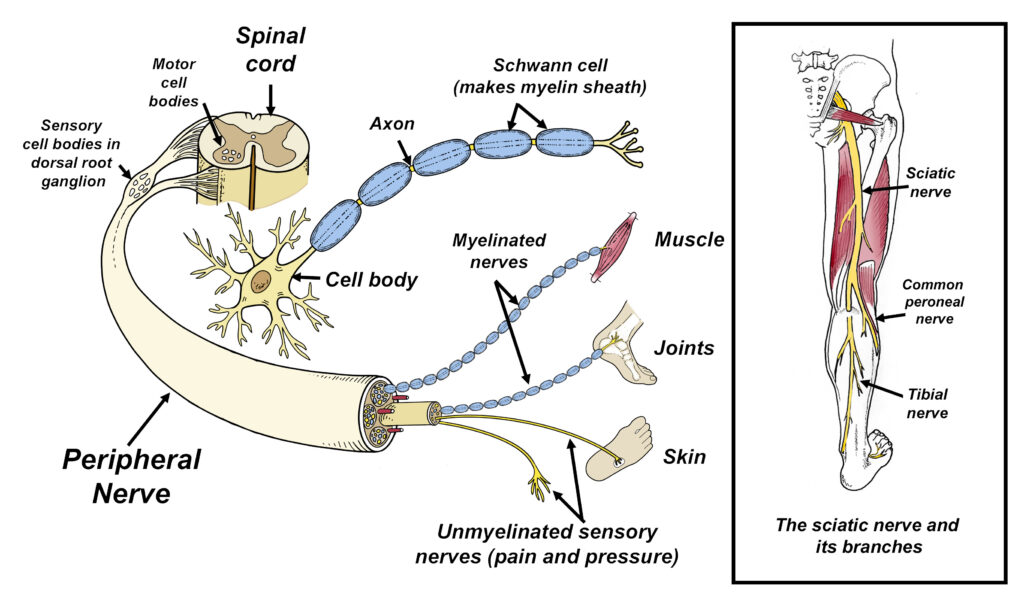
Fig. 1. Peripheral nerves branch off the spinal cord and are comprised of a cell body and its axon, which may or may not be covered with the myelin sheath. This sheath is produced by specialized cells called Schwann cells, which play an important role in maintaining the overall health of the axon. In addition to supporting the axon with nutrients, the myelin sheath also acts as an insulator that increases the speed in which electric currents traverse the nerve. The peripheral nerves leading to muscles are called motor nerves, while the different types of sensory nerves go to almost every cell in the body. In the low back, peripheral nerves group together to form the sciatic nerve, which is the longest nerve in the body. For reasons that are poorly understood, peripheral neuropathies cause the nerves to break down, damaging the Schwann cells, axons, and/or cell bodies.
While any peripheral nerve may be involved, the sensory nerves that go to the feet are the most prone to developing neuropathy. Part of the reason these nerves are preferentially targeted is because they are so long: a single nerve that goes to the bottom of the foot can be over 3 feet long, and the nerve’s axon can be 20,000 times longer than the cell body (2). Because the cell body assists with nourishing and maintaining the axon, providing nutritional and metabolic support to such a long structure can be difficult under ideal conditions. The connection between nerve length and peripheral neuropathy is evidenced by the fact that tall people are significantly more likely to get neuropathy than their shorter peers (3).
Another reason sensory nerves are preferentially targeted is because their cell bodies are located outside the central nervous system in the dorsal root ganglion, which exposes them to a wide range of chemical and metabolic irritants (4) (refer back to figure 1). In contrast, because the cell bodies of motor nerves are located within the central nervous system, they benefit from the protection provided by the blood-brain barrier, which constantly filters out harmful molecules and chemicals creating a relatively sterile environment. Even beneficial drugs such as antibiotics and chemotherapy agents are unable to cross the blood-brain barrier.
The neurological symptoms associated with peripheral neuropathies are determined by the specific sensory nerves involved. If the small diameter unmyelinated nerves are affected, typical symptoms will include pain, tingling, and numbness. If the large diameter unmyelinated nerves are involved, people usually present with impaired coordination and/or decreased sensation in the soles of their feet. Impaired sensation is extremely dangerous as it strongly correlates with future falls and increases the risk of cuts, foot ulcerations and major amputations.
Some of the more common causes for the development of this disease include various autoimmune disorders, alcoholism, kidney disease, exposure to toxins, vitamin deficiencies, and certain medications. Chemotherapy drugs are notorious for producing peripheral neuropathy. While all of these conditions increase the risk of neuropathy, far and away the greatest risk factor for developing peripheral neuropathy is diabetes. An estimated 20 million Americans currently have diabetes-related neuropathy, and that number is expected to double as more Americans develop pre-and type II diabetes (5). Worldwide, there are approximately 200 million people with diabetes-related peripheral neuropathy and up to 50% of people with peripheral neuropathy are asymptomatic, which allows the condition to progress unnoticed (6). According to Feldman et al. (4), the neuropathic complications associated with diabetes places a tremendous burden not just on individuals affected, but society as a whole. The authors emphasize that there is a pressing need to understand the mechanisms underlying diabetic neuropathy and develop effective interventions to treat and prevent this disabling condition. Unfortunately, despite 50 years of intense research, pharmacological interventions for peripheral neuropathy remain relatively ineffective (4). This is especially true for chemotherapy induced peripheral neuropathies, as to this day there are still no FDA-approved drugs for the treatment or prevention of this common type of neuropathy (7).
Because pharmacological interventions have been unable to appreciably improve the quality of life for those suffering with peripheral neuropathy, researchers have begun focusing on alternative treatments, such as exercise, nerve glide stretches, vibration therapy, textured insoles, and nutrition. Of these interventions, exercise interventions have been studied most extensively. In one of the first papers to evaluate the efficacy of exercises in the management of peripheral neuropathy, researchers from the University of Kansas had 17 peripheral neuropathy patients perform a 10-week program of aerobic and strengthening exercises (8). At the start and stop of the study, the authors evaluated pain levels using visual analog scales, performed nerve conduction velocities, and measured the density and branching patterns of cutaneous nerves located in skin taken from biopsies. Participants performed light aerobic exercises on devices such as elliptical trainers and bicycles and did a conventional strengthening protocol targeting all of the major muscle groups. The exercises were performed at a moderate intensity. At the end of the 10-week study, the exercise group experienced significant reductions in pain and neuropathic symptoms, and they actually grew additional cutaneous nerve fibers following the intervention. This latter finding was consistent with prior research confirming that exercise can increase the number of sensory nerve fibers present in the skin (9) (Fig. 2), which is important as the regeneration of cutaneous nerve fibers correlates with improved clinical outcomes (10).
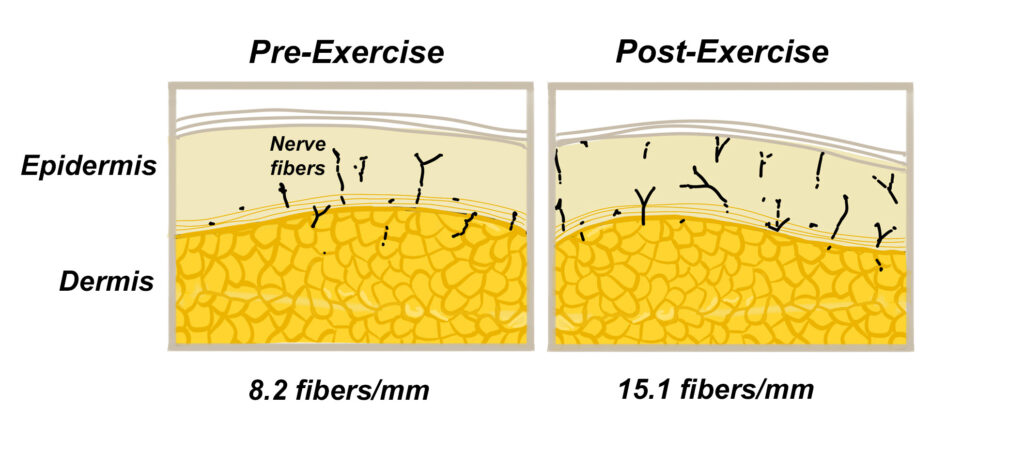
Fig. 2. A 40-fold magnification of skin shows how 12-months of exercise produces a significant increase in the number of cutaneous nerve fibers in the epidermis. Redrawn from photographs in Smith et al. (9).
The findings of these early research studies have been corroborated by more recent research confirming that exercise interventions can delay both the onset and progression of both diabetes and diabetes-related peripheral neuropathies (11-19). Exercise intervention has also been proven to be effective when managing chemotherapy-induced peripheral neuropathy (20,21). One study found that just 8 weeks of resistance and balance training produced statistically significant reductions in patient reported pain, dynamic balance, range of motion, and quality of life in cancer survivors (21). The positive effects of exercise have been related to improved glycemic control, increased blood flow, enhanced neurotrophic support and suppression of pro-inflammatory responses. According to Holmes et al. (22), a combination of aerobic and strength training gets the best results, and my favorite program is outlined in figure 3. This routine is typically done 3 times per week for 12 weeks, and a video of the entire program is available at humanlocomotion.com.
Figure 3. Peripheral neuropathy exercise routine.
WARM-UP EXERCISES: Choose between stationary or recumbent bike, elliptical or treadmill walking on incline. Start with 10 minutes and build up to 15-20 minutes.
BALANCE EXERCISE: With shoes on, stand with one leg on foam pad with eyes open for 3 minutes. If difficult, consider wearing Balance Buttons (see figure 6 below). As balance improves, try closing eyes on occasion, making sure you’re near a stable structure in case you lose balance.
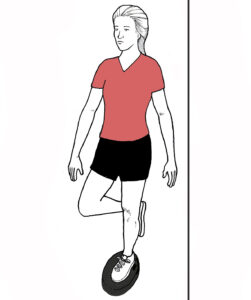
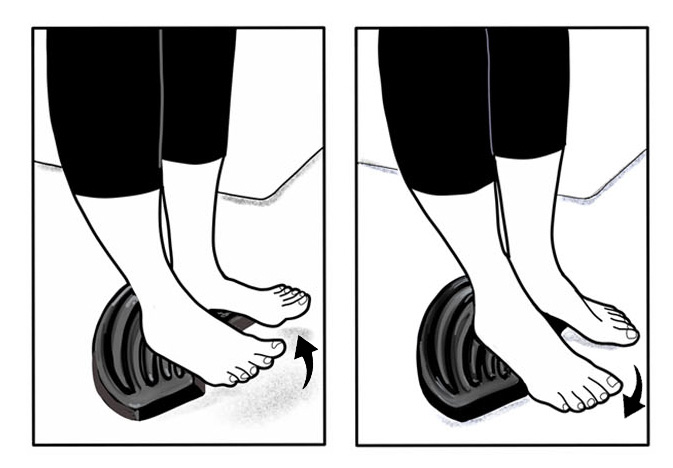
TOEPRO EXERCISES: Place toes on front of ToePro and raise then lower heels while tilting your heels in and out (arrows). While raising heels, push down vigorously with your toes (A). Do 3 sets of 25 reps. If difficult, perform next to a table and push down with hands. As you get stronger consider holding a weight in one hand.
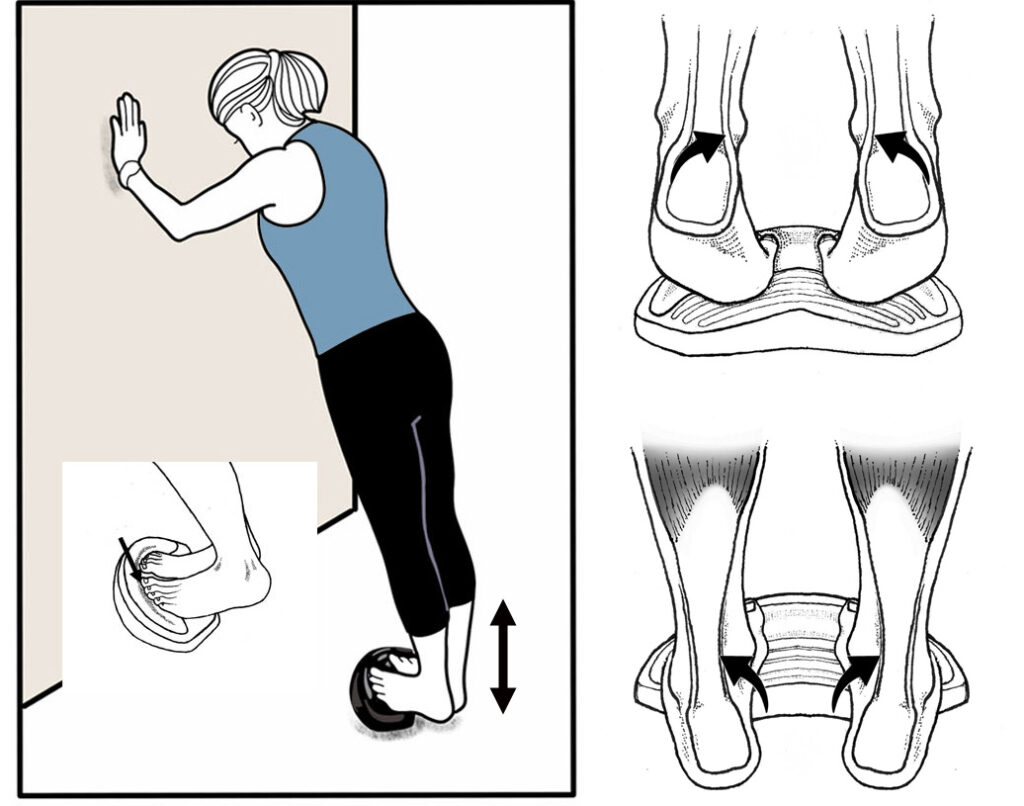
ANKLE DORSIFLEXION: Stand with heels behind top crest of ToePro with your forefeet on the floor. Do 2 sets of 15 reps raising and lowering your forefeet and toes. Build to 3 sets of 15 reps.
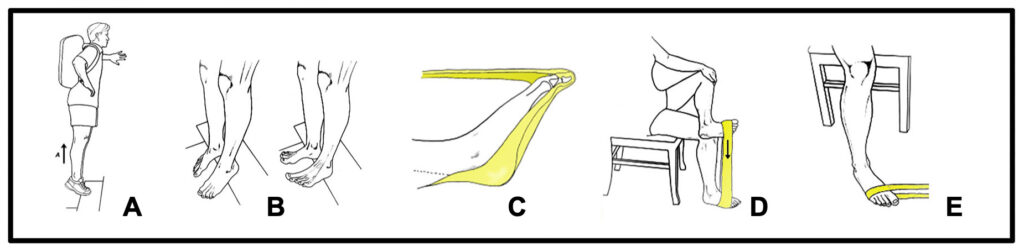
ALTERNATE TOEPRO EXERCISES: To strengthen your feet without using a ToePro, do 2 sets of 15 reps of each of the above exercises. A) Heel raises on stair. B) Toe raises on stair. C) Toe pushdowns with elastic band. D) Invertor elastic band exercise and E) Evertor exercise. To increase resistance, add weight with stair exercises and/or switch to heavier resistance band.
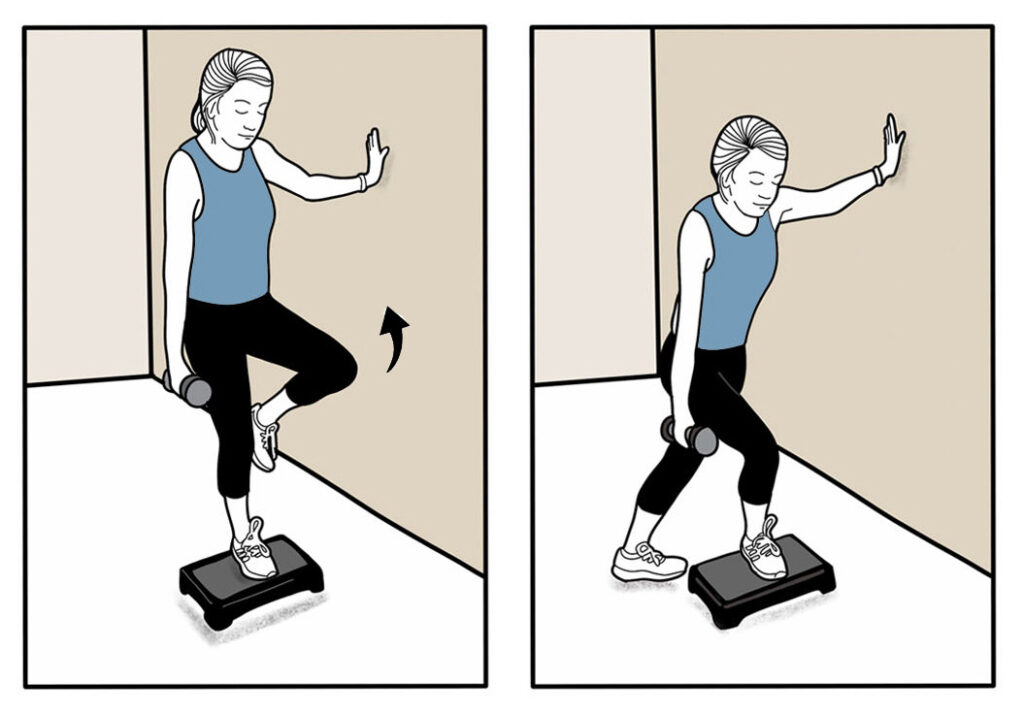
HIP AND KNEE EXTENSORS: Stand on a 4-inch step and do a curtsy step-up while holding onto a wall. Start with 2 sets of 15 reps on each leg. As you get stronger, increase to 3 sets of 15 reps while holding onto a weight.
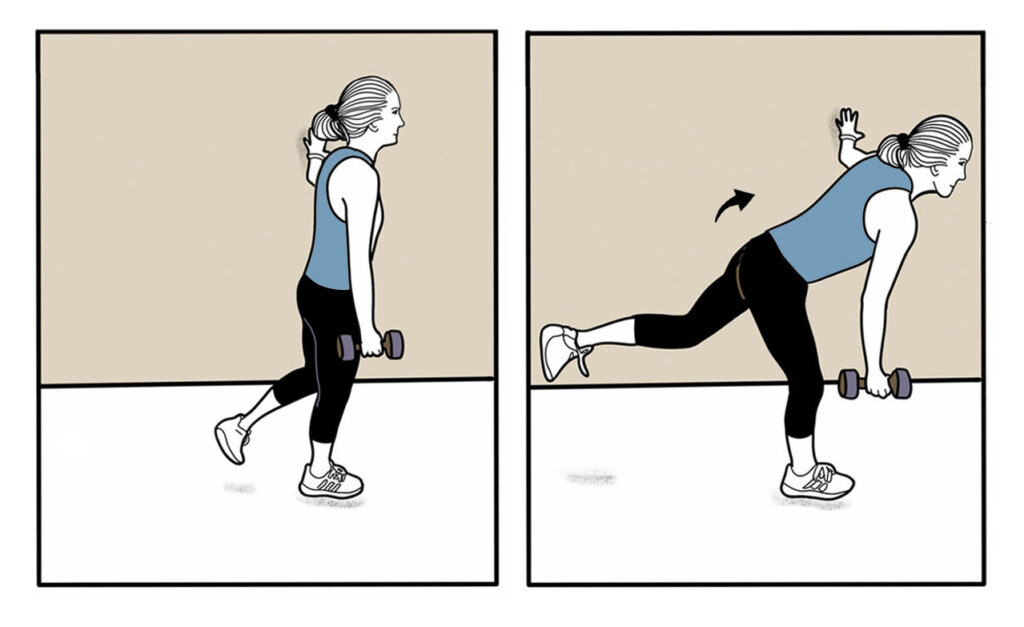
HAMSTRINGS: While holding onto wall for support, pivot forward at the hip while standing on one leg. Do 2 sets of 15 reps and maintain a straight spine while performing these exercises. Progress to 3 sets of 15 reps while holding a weight.
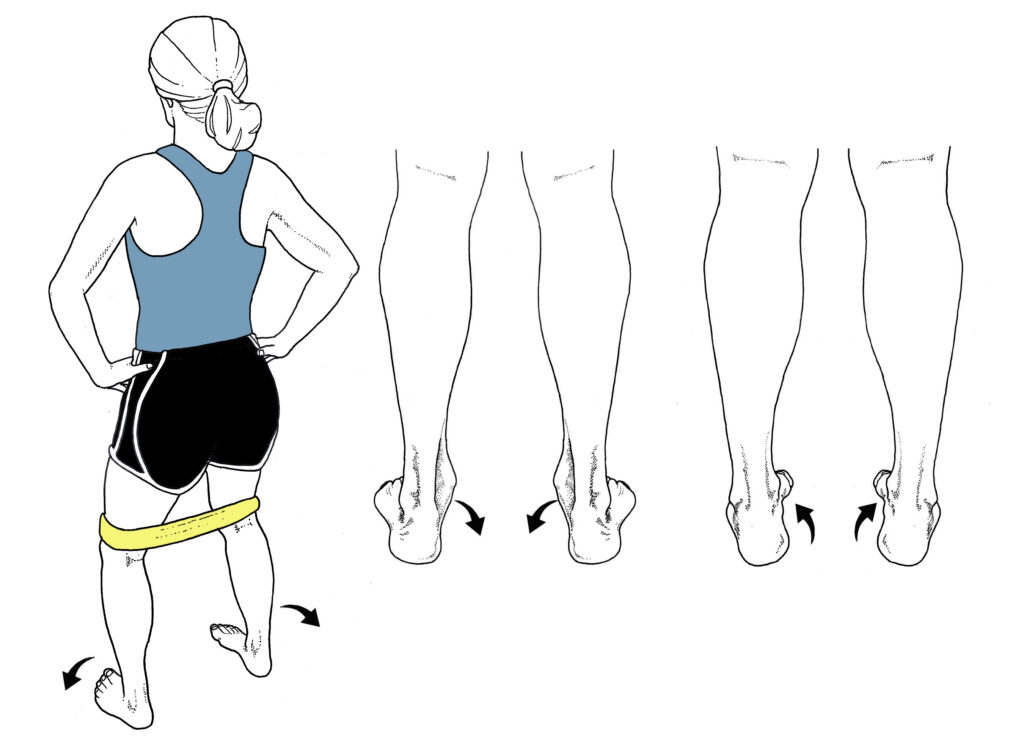
HIP ABDUCTORS: Lie on your side with your top leg hanging off of a workout bench (or any soft surface) with your toes pointing down. Raise your foot towards the ceiling while rotating your leg so your toes point up slightly. Do 2 sets of 15 reps on each leg, gradually building up to 2 sets of 25 repetitions.
ANKLE INVERTORS/EVERTORS: Place a 2:1 Rockboard near a wall with your foot positioned over the abrasive strip so that your arch points towards the long side of the board (A). While keeping your hip and knee straight, use your ankle to rotate the rockboard so the edges touch the ground on all sides. Do 30 clockwise rotations with your left foot and 30 counterclockwise rotations with your right foot. Repeat this 2 times. Gradually build up to 3 sets of 30 reps on each foot.
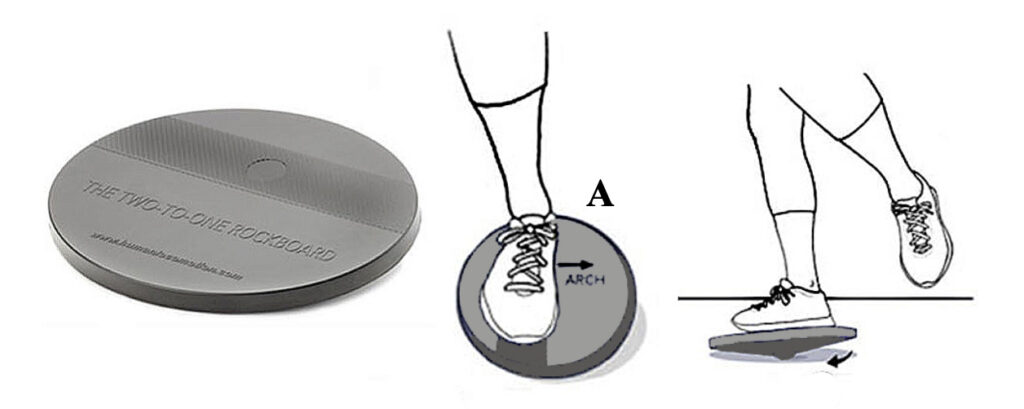
ALTERNATE EXERCISES FOR THE 2:1 ROCKBOARD: If you do not have a rockboard, just stand with your feet separated slightly with an elastic band wrapped around your knees. In this position, raise and lower your arches as far as possible (A and B). Do 2 sets of 15 repetitions and gradually build to 3 sets of 15 reps.
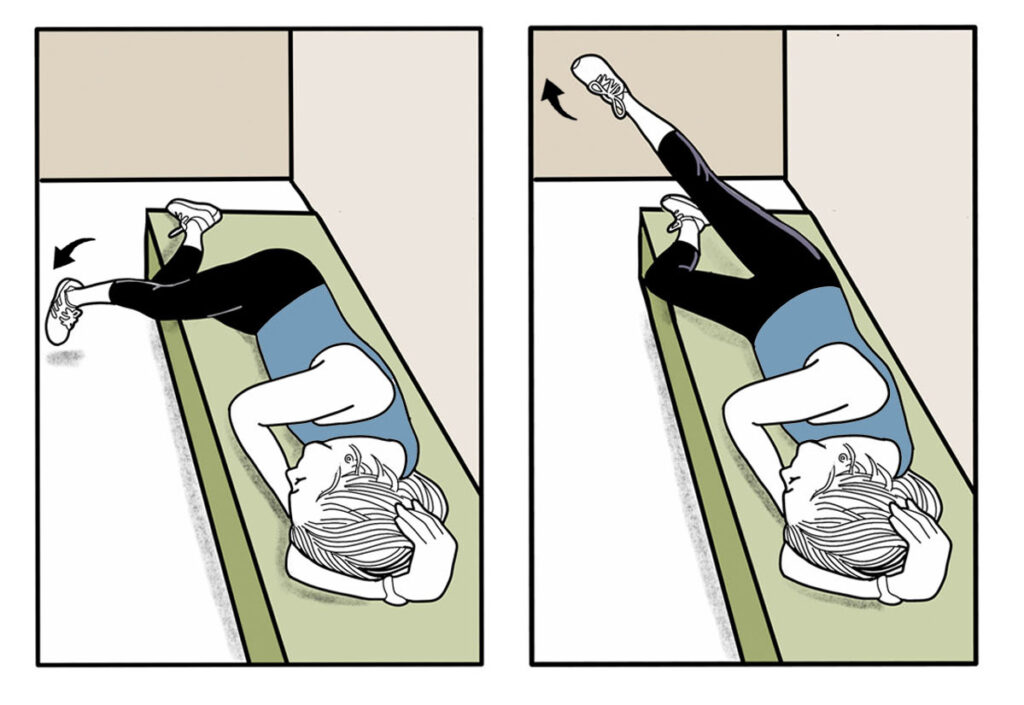
In addition to exercise, gentle nerve glide stretches have also been shown to improve nerve function in people with neuropathy. Performing range of motion and specific nerve glide stretches just 3 times daily for 10 minutes appreciably reduced nerve pain while improving strength and sensation in patients suffering from chemotherapy-induced peripheral neuropathy (23) (Fig. 4). The authors claim that nerve glide exercises improve nerve function by decreasing neural edema as stretching the nerve enhances the flow of nutrients through the axon. Other authors speculate that nerve glide techniques work because they loosen adhesions that restrict proper nerve motion. Regardless of the mechanisms, gently moving peripheral nerves back and forth can reduce pain and improve function. In fact, many of the exercises in the peripheral neuropathy strengthening program were included because they mobilize branches of the sciatic nerve, which is the longest nerve in the body and is almost always affected by peripheral neuropathy.
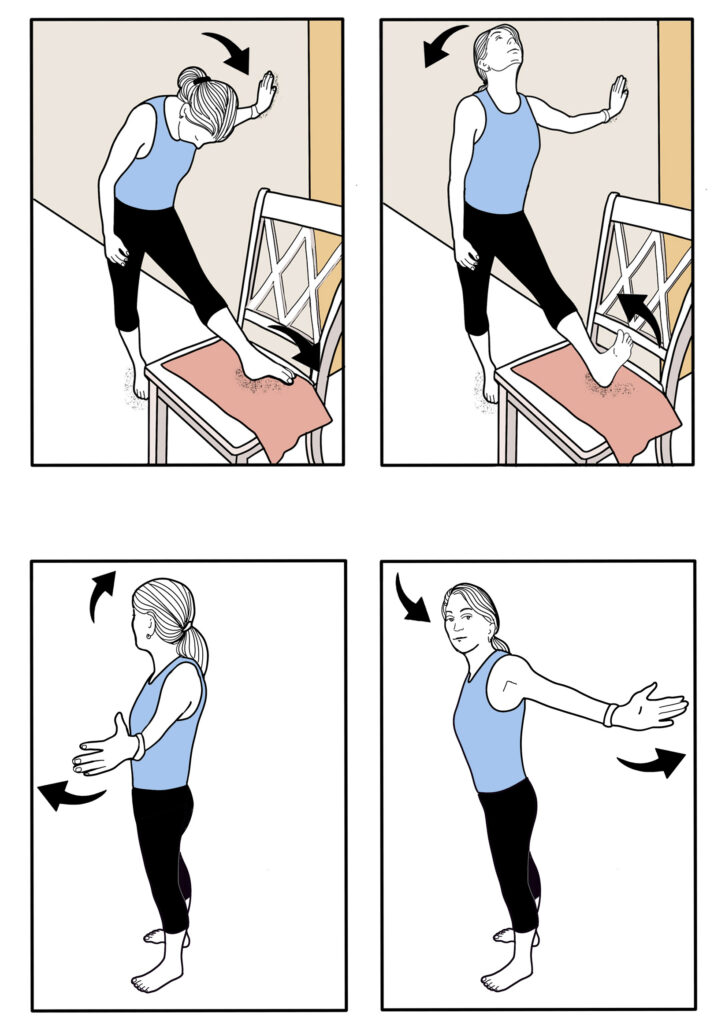
Fig. 4. Common nerve glide techniques. Alternating movements of the head and extremities moves the long peripheral nerves back and forth, stretching adhesions between nerve fibers and neighboring connective tissues that can negatively affect function. Nerve glides also improve the flow of nutrients through the nerves’ axons. A series of nerve glides that effectively improve function in the upper extremity are described by Hammond et al. (23).
Perhaps the most interesting technique to improve nerve function in people with peripheral neuropathy is the application of vibration stimulus to the soles of the feet. What makes this approach so appealing is that while compliance with exercise intervention is notoriously low, performing 3, 10-minute sessions of vibration therapy is pretty much the easiest intervention you can do short of taking a pill. In 2021, researchers from Iran performed a single subject study in which the bottoms of both feet were vibrated at a frequency of 63 Hz for 10 minutes (24). This was repeated on 5 different days. At the end of the intervention, there was an astonishing 63% reduction in pain severity, with measurable increases in skin temperature, and marked improvements in blood flow and balance. The outcomes of this study were similar to prior research showing that an 8-week intervention of foot vibration in 12 patients with diabetic peripheral neuropathy improved conduction velocities of both the sural and peroneal nerves (which supply sensation to the feet), increased postural stability, and produced significant reductions in pain and tingling (25). Unlike whole body vibration, which is expensive and often has unwanted side effects (26), local vibration can be applied with inexpensive home massage units (Fig. 5) and the only requirement is that the frequency be set at 40-60 Hz.
Fig. 5. The Peanut Home Massager oscillates at a frequency of 55 cps and costs about $40. The two heads of this device allow you to roll both feet over the top of the massage unit at the same time. Because of the intensity of the vibration, the massage unit is typically placed on a pillow positioned on the floor. This device can also be used as a foam roller to massage pretty much any muscle in the body, and recent research shows these devices can accelerate muscle repair following heavy exercise (31).

As far as the potential for injury goes, one of the biggest problems with peripheral neuropathy is that it often results in impaired sensation along the bottom of the feet. Even a slight reduction in sensation can greatly increase the risk of falling and/or developing foot ulcerations. To prevent this, people with peripheral neuropathy presenting with impaired sensation should consider wearing textured insoles (Fig. 6). These insoles have special elevations that amplify cutaneous sensation, like a hearing aid amplifies sound. The nice thing about textured insoles like the Balance Buttons is that they are inexpensive, and you know right away whether or not they will be helpful: as soon as you put them in your shoes, you should notice an immediate improvement in balance. The only downside is that they only work while you are wearing them, and they need to be replaced every 6 to 9 months.
Surprisingly, one of the most overlooked aspects of managing peripheral neuropathy is nutrition. Although there are thousands of articles detailing specific vitamins and nutrients you should take to manage peripheral neuropathy, one of the most effective dietary approaches is to consume a low-inflammatory diet. In a 15-year study of more than 140,000 people, researchers from China showed that people consuming low-inflammation foods were 74% less likely to develop type II diabetes (27). Decreasing the risk of type II diabetes significantly reduces the risk of developing diabetic peripheral neuropathy.
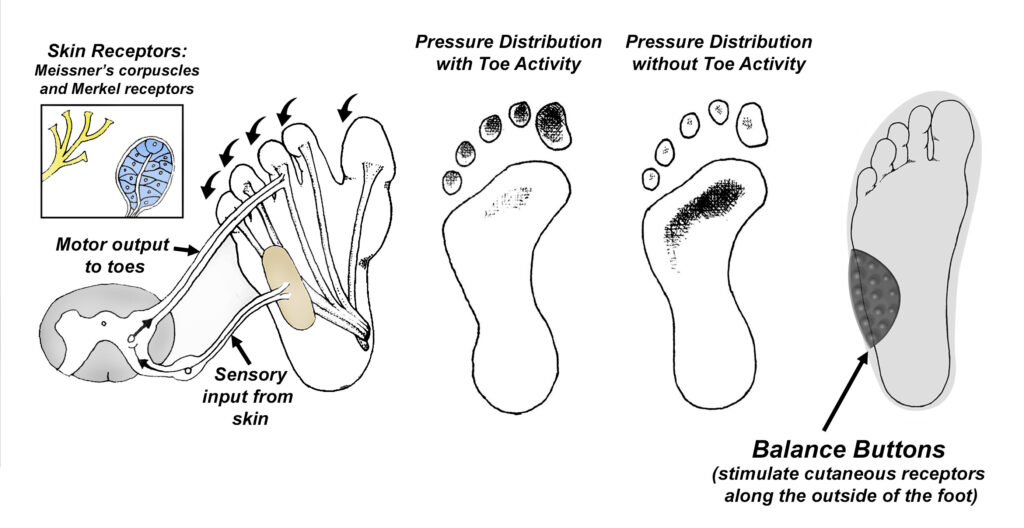
Fig. 6. Textured insoles. The bottoms of our feet are loaded with thousands of specialized cutaneous receptors that tell our central nervous system the precise location of our balance point. Recent research shows the vast majority of these receptors are located along the outer sides of our feet (X) (32). When these cutaneous receptors are stimulated, their sensory nerves produce an immediate reflex contraction of the toe muscles, which greatly reduces pressure in the center of our forefeet: every pound of downward force generated by the toes offloads the central forefoot by the same amount. This reflex activity protects the center of the forefoot from developing skin ulcerations. Reflex toe activity also plays an important role in preventing falls, as downward pressure of the toes keeps us stable as we lean forward. Because peripheral neuropathies impair transmission along sensory nerves, it can take as much as 80% more pressure to stimulate these cutaneous nerves. Without important information supplied by these receptors, the risks of skin ulcerations and falls skyrockets. To counter the neuropathy-induced impaired sensation, textured insoles like Balance Buttons have progressively larger elevations along the top surface that stimulate cutaneous receptors even in cases of moderate to advanced nerve damage. If you inadvertently shift your weight too far to the outside of the foot while wearing Balance Buttons, the elevations compress and stimulate even impaired cutaneous receptors, producing the reflexive muscular responses necessary to remain upright and avoid injury.
The most promising supplement for the management of peripheral neuropathy is α-lipoic acid. The mechanism of action of this important nutrient is that α-lipoic acid reduces oxidative stress and improves nerve blood flow. The typical dosage ranges from 600 to 1800 mg, and α-lipoic acid has recently been labeled by the FDA as safe and effective for the treatment of painful diabetic peripheral neuropathy (28). A meta-analysis of 4 randomized, placebo-controlled trials with over 1200 participants with painful neuropathy showed that alpha-lipoic acid improves neuropathic symptoms 24% (29). These results are similar to the reduced symptoms associated with many pharmacological agents, such as gabapentin and SSRI’s.
Given the diverse mechanisms responsible for the development of peripheral neuropathy, it is clear that alternative interventions can play a pivotal role in the management of this complex and often painful condition. Unlike many pharmacological interventions, the alternative interventions described in this article are safe, effective, and inexpensive. Research shows these techniques can reduce pain, enhance balance, and improve the overall quality of life for those afflicted with this painful condition. The only downside is that because exercise and stretching routines require a substantial commitment of time and effort, compliance is often notoriously low. This can be addressed by decreasing the intensity, sets, repetitions, and/or frequency of the prescribed exercises. The same is true for the nerve glide stretching techniques, as the ideal duration of the stretching programs has not been determined. Compliance with vibration therapy and/or textured insoles is typically not an issue, as they are incredibly simple to incorporate and often provide rapid relief, which is a great motivator. In contrast, compliance with nutritional modifications can be difficult, but when you consider that healthy diets have been shown to increase lifespan by as much as 10 years (30), it should be an important part of everyone’s healthcare routine.
References:
- Hicks C, Wang D, Windham B, et al. Prevalence of peripheral neuropathy defined by monofilament insensitivity in middle-aged and older adults in two US cohorts. Scientific Reports. 2021;27;11:19159.
- Wang, J, Medress, Z, Barres, B. Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012; 196:7–18.
- Kote G, Bhat A, Thajuddeen K, et al. Peripheral insensate neuropathy-is height a risk factor? J Clin Diag Research. 2013;7:296.
- Feldman, Eva L., et al. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017:1296-1313.
- Menke, A., Casagrande, S., Geiss, L., and Cowie, C.C.). Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314:1021–1029 .
- American Diabetes Association Professional Practice Committee. 12. Retinopathy, neuropathy, and foot care: standards of care in diabetes—2024. Diabetes Care 2024;47(Suppl 1):S231–S243..
- Kleckner, Ian R., et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Supportive Care in Cancer 2018; 26:1019-1028.
- Kluding, Patricia M., et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. Journal of Diabetes and its Complications. 2012;26.5:424-429.
- Smith, A. G., Russell, J. W., Feldman, E. L., Goldstein, J., Peltier, A., Smith, S., et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–1299.
- Baskozos, Georgios, et al. Molecular and cellular correlates of human nerve regeneration: ADCYAP1/PACAP enhance nerve outgrowth. Brain. 2020;7: 2009-2026.
- Zaccaria S, Di Perna P, Giurato L, Pecchioli C, Sperti P, Arciprete F,et al. Diabetic polyneuropathy and physical activity in type 1 diabetes mellitus: a cross-sectional study. J Clin Med. 2023;12:6597.
- Kristensen FPB, Sanchez-Lastra MA, Dalene KE, Del Pozo Cruz B, Ried-Larsen M, Thomsen RW, et al. Leisure-time physical activity and risk of microvascular complications in individuals with type 2 diabetes: a U.K. Biobank study. Diabetes Care. 2023;46:1816–24.
- Streckmann F, Balke M, Cavaletti G, et al. Exercise and neuropathy: systematic review with meta-analysis. Sports Med. 2022 May;525:1043–65.
- Kanaley JA, Colberg SR, Corcoran MH, Malin SK, Rodriguez NR, Crespo CJ, et al. Exercise/physical activity in individuals with type 2 diabetes: a consensus statement from the American College of Sports Medicine. Med Sci Sports Exerc. 2022;1:353–68.
- Amanat S, Ghahri S, Dianatinasab A, Fararouei M, Dianatinasab M. Exercise and type 2 diabetes. Adv Exp Med Biol. 2020;1228:91–105.
- Jenkins DW, Jenks A. Exercise and diabetes: a narrative review. J Foot Ankle Surg. 2017;56:968–74.
- Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia. 2016;59:2527–45.
- Naylor LH, Davis EA, Kalic RJ, Paramalingam N, Abraham MB, Jones TW, et al. Exercise training improves vascular function in adolescents with type 2 diabetes. Physiol Rep 2016;4:e12713
- Akhtar S. Diabetes-induced peripheral neuropathy: Is prescribing physical exercise the answer? Biomolecules and Biomedicine. 2024 Jan 11.
- Nuñez de Arenas- Arroyo S, Cavero- Redondo I, Torres- Costoso A, Reina- Gutiérrez S, Lorenzo- García P, Martínez- Vizcaíno V. Effects of exercise interventions to reduce chemotherapy- induced peripheral neuropathy severity: A meta-analysis. Scand J Med Sci Sports. 2023;00:1-14.
- McCrary, J. Matt, et al. “Exercise-based rehabilitation for cancer survivors with chemotherapy-induced peripheral neuropathy.” Supportive Care in Cancer 2019;27:3849-3857.
- Holmes CJ, Hastings MK. The application of exercise training for diabetic peripheral neuropathy. Journal of Clinical Medicine. 2021 Oct 28;10(21):5042.
- Hammond E, Pitz M, Steinfeld K, et al. An exploratory randomized trial of physical therapy for the treatment of chemotherapy-induced peripheral neuropathy. Neurorehabilitation and Neural Repair. 2020;34: 235-246.
- Sabziparvar M, Naghdi S, Ansari N, et al. Local plantar vibration for the treatment of diabetic neuropathy: a case report. Journal of Diabetes & Metabolic Disorders. 2021;20: 2115-2119.
- Stambolieva K, Petrova D, Irikeva M. Positive effects of plantar vibration training for the treatment of diabetic peripheral neuropathy: a pilot study. Somatosensory & Motor Research. 2017;34:129-33.
- Seidel H. On the relationship between whole-body vibration exposure and spinal health risk. Ind Health. 2005;43:361–377.
- Yang R, Lin J, Yang H et al. A low-inflammatory diet is associated with a lower incidence of diabetes: role of diabetes-related genetic risk. BMC Medicine. 2023;21.1:483.
- Ardeleanu V, Toma A, Pafili K, et al. Current pharmacological treatment of painful diabetic neuropathy: A narrative review. Medicina. 2020;56.1: 25.
- Ziegler D, Nowak H, Kempler P, et al. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: A meta-analysis. Diabet. Med. 2014 ; 21:114–121.
- Fadnes LT, Økland JM, Haaland ØA, et al. Estimating impact of food choices on life expectancy: a modeling study. PLoS Med. 2022;19, e1003889.
- Chang Y, Tseng W, Chiu C, et al. The effects of a preconditioning vibration rolling warm‐up on multidirectional repeated sprinting‐induced muscle damage. European Journal of Sport Science. 2024;24.1: 36-44.
- Strzalkowski N, et al. Cutaneous afferent innervation of the human foot sole: what can we learn from single-unit recordings? J Neurophysiol. 2018;120:1233–1246,.33.
